Abstract
Background: Patients (pts) with lymphoma report impaired HRQoL as a result of disease- and treatment-related symptoms (Hlubocky FJ, et al. Leuk Lymphoma 2013). However, there are limited dynamic data for quality of life using patient-reported outcome (PRO) measures from large prospective clinical trials in pts with previously untreated DLBCL. The Phase III POLARIX study (NCT03274492) demonstrated superior progression-free survival (PFS) and a similar safety profile with Pola-R-CHP vs R-CHOP in pts with previously untreated DLBCL (Tilly H, et al. N Engl J Med 2022). In this analysis, PROs were used to measure physical functioning, fatigue, lymphoma-related symptom scores, and treatment-related symptom scores to fully characterize the pt experience in POLARIX.
Methods: In total, 879 pts with previously untreated DLBCL were randomized 1:1 to Pola-R-CHP (n=440) or R-CHOP (n=439). Lymphoma symptom scores were assessed using the Functional Assessment of Cancer Therapy-Lymphoma Subscale (FACT-LymS; Hlubocky FJ, et al. Leuk Lymphoma 2013). Physical functioning, fatigue, constipation, diarrhea, nausea, and vomiting were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life-Core 30 questionnaire (EORTC QLQ-C30; Aaronson NK, et al. J Natl Cancer Inst 1993). Questionnaires were administered to patients without progression during treatment on Day 1 of Cycles 1 (baseline), 2, 3, and 5, at end of treatment (EOT), biannually for 2 years after EOT, then annually for the following 3 years. Questionnaire compliance and summary statistics at each assessment time point were estimated. Percentage of pts with clinically meaningful improvements, worsening, and no changes from baseline for physical functioning (≥7-point increase, ≥10-point decrease, and in between, respectively), fatigue (≥9-point increase, ≥10-point decrease, and in between, respectively), and lymphoma symptom scores (≥3-point increase, ≥3-point decrease, and in between, respectively) were reported based on validated thresholds (Aaronson NK, et al. J Natl Cancer Inst 1993; Hlubocky FJ, et al. Leuk Lymphoma 2013). Changes over time in physical functioning, fatigue, lymphoma symptom scores, and treatment-related symptom scores for constipation, diarrhea, nausea and vomiting were assessed.
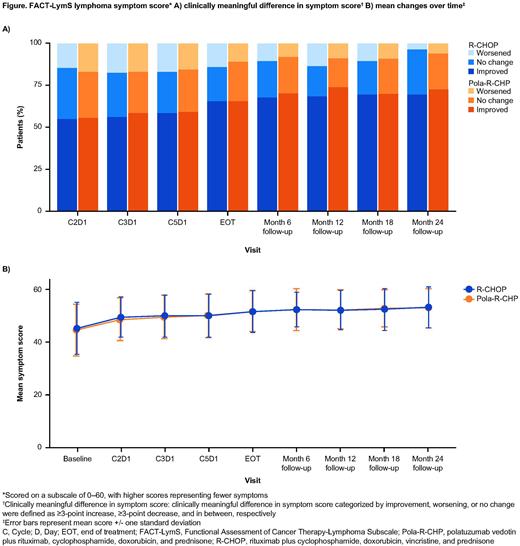
Results: Questionnaire response rates were high (>80%) throughout the study. At baseline, scores for lymphoma symptoms, physical functioning, and fatigue were similar in both treatment arms; levels of constipation, diarrhea, nausea, and vomiting were similarly low. Both Pola-R-CHP and R-CHOP induced a rapid improvement in lymphoma symptom scores in most pts after Cycle 1 (pts with clinically meaningful improvement at any time point: 82.3% vs 81.3%, respectively), and this was sustained with improvement plateauing by the first assessment after EOT (Figure). Improvements in physical functioning and fatigue were similar with Pola-R-CHP vs R-CHOP (pts with clinically meaningful improvement at any time point in physical functioning: 42.4% vs 39.6%; fatigue: 74.8% vs 68.2%, respectively); improvements plateaued by the first assessment after EOT. Between Cycle 2 and EOT, diarrhea symptoms were more frequently reported in pts treated with Pola-R-CHP (range: 17-34%) than with R-CHOP (range: 18-24%), and constipation symptoms were reported more frequently with R-CHOP (range: 21-42%) than with Pola-R-CHP (range: 23-35%); diarrhea and constipation symptoms returned to baseline levels by EOT. Nausea and vomiting symptoms were reported infrequently in both treatment arms (nausea: range 12-33% in Pola-R-CHP, 11-30% in R-CHOP; vomiting: range 3-11% in Pola-R-CHP, 4-10% in R-CHOP) and returned to baseline levels at EOT.
Conclusions: Both regimens led to rapid and sustained improvements in symptoms and improved HRQoL. Lymphoma symptom scores were improved in the majority of pts after Cycle 1, with improvement maintained in most pts. Physical functioning and fatigue scores were similar at baseline between arms, and improved during and after treatment. During chemotherapy, treatment-related symptoms (i.e. nausea, vomiting, constipation, diarrhea) were observed in a minority of pts, with a return to baseline after treatment completion. These data may represent a benchmark for patient-reported HRQoL in frontline DLBCL in the modern era.
Disclosures
Trněný:Zentiva: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding. Morschhauser:Genmab: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Janssen: Speakers Bureau; Miltenyi: Membership on an entity's Board of Directors or advisory committees; Allogene therapeutics: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Salles:Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria; AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees. Reagan:Genentech: Research Funding; Seagen: Research Funding; Kite, a Gilead Company: Consultancy; Caribou Biosciences: Consultancy. Hertzberg:Takeda: Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Roche, Takeda, Otsuka, Beigene, Gilead, Janssen, Novartis, Mundipharma: Honoraria, Membership on an entity's Board of Directors or advisory committees. Smolewski:Medical University of Lodz: Current Employment, Ended employment in the past 24 months; Roche Poland: Honoraria, Research Funding; Takeda: Honoraria, Research Funding. Thieblemont:Novartis: Consultancy, Honoraria, Other: Travel Support, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support; Incyte: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other: Travel Support; Celgene: Consultancy, Honoraria, Other: Travel Support; AbbVie: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: Travel Support; Bristol Myers Squibb: Consultancy, Honoraria, Other: Travel Support. Hu:Incyte: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech/Roche: Research Funding. Fonseca:Florida Cancer Specialists: Current Employment; Morphosys: Honoraria; Pfizer: Honoraria; Amgen: Honoraria. Kim:Takeda: Honoraria; Beigene: Research Funding; Sanofi: Research Funding; Kyowa-kirin: Research Funding; Boryong: Research Funding; Donga: Research Funding. Martelli:Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eusapharma: Membership on an entity's Board of Directors or advisory committees; Sandoz: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Mehta:University of Alabama: Current Employment; Gilead: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; Pharmacyclics: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Research Funding, Speakers Bureau; Morphosys/Incyte: Consultancy, Speakers Bureau; TG Therapeutics: Consultancy, Research Funding; Kyowa Kirin: Consultancy, Speakers Bureau; Novartis: Consultancy; BMS: Consultancy, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; Takeda: Research Funding; fortyseven inc/Gilead: Research Funding; Juno Pharmaceuticals/BMS: Research Funding; Celgene/BMS: Research Funding; Innate Pharmaceuticals: Research Funding; Affimed: Research Funding; Merck: Research Funding; Kite/Gilead: Research Funding; Roche/Genentech: Research Funding; I-MAB: Research Funding. Campinha-Bacote:Genentech/Roche: Current Employment, Current equity holder in publicly-traded company. Yan:Hoffmann-La Roche: Current Employment, Current equity holder in publicly-traded company. Hirata:Genentech, Inc.: Current Employment; Roche: Current holder of stock options in a privately-held company. Sugidono:Genentech, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Lee:Genentech: Current Employment; Roche: Current equity holder in publicly-traded company. Sharman:Pharmacyclics LLC, an AbbVie Company: Honoraria; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Araris Biotech AG: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Lilly: Consultancy, Honoraria, Research Funding; Merck: Consultancy; TG Therapeutics: Consultancy, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Genentech: Consultancy; BMS: Consultancy, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding.
OffLabel Disclosure:
Polatuzumab vedotin (Polivy) is a CD79b-directed antibody-drug conjugate indicated in combination with bendamustine and a rituximab product for the treatment of adult pts with relapsed or refractory DLBCL, not otherwise specified, after at least two prior therapies. Rituximab (Rituxan) is a CD20-directed cytolytic antibody indicated for the treatment of adult pts with: relapsed or refractory, low grade or follicular, CD20-positive, B-cell NHL as a single agent; previously untreated follicular, CD20-positive, B-cell NHL in combination with first-line chemotherapy (chemo) and, in pts achieving a CR or PR to a rituximab product in combination with chemo, as single-agent maintenance therapy; non-progressing (including stable disease), low-grade, CD20-positive, B-cell NHL as a single agent after first-line CVP chemo; previously untreated diffuse large B-cell, CD20-positive, NHL in combination with CHOP or other anthracycline-based chemo regimens; previously untreated and previously treated CD20-positive CLL in combination with fludarabine and cyclophosphamide.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal